|
MACROBEL project goals and database
The general aim of this research project is to deliver a substantial contribution to the knowledge of the
long-term variability within the biodiversity and density patterns of the macrobenthos and its relation to
anthropogenic activities.
In detail:
- Spatial distribution and long-term variation (on a large scale) of the macrobenthos
- Making of a distribution atlas of the macrobenthos on the BCS for the periods 1977-1986 and
1994-2001 (by collating already existing data with newly gathered ones)
- Evaluation of long-term variation of the macrobenthos on the BCS (1977-1986 versus 1994-2001)
- Evaluation of the different influences on macrobenthos, caused by anthropogenic activities
- Detailed long-term variation of the macrobenthos on the BCS
- Detailed study of the long-term variation of the macrobenthos on the BCS in 4 stations stressed by
anthropogenic activities
- Detailed evaluation of the influences of a selection of anthropogenic activities on macrobenthos of
the BCS (study on causal connections)
MACROBEL
The database is derived from an existing relational Access database MACRODAT (developed by Drs. Tim Deprez, SMB),
which combines taxonomy, geography, and biotic and abiotic data. MACROBEL will contain a full compilation of all
available macrobenthos data of the BCS from the periods 1976-1986 en 1994-2001 and will be hosted by VLIZ
(Flanders Marine Institute).
Quality control identifications
Overview of used identification manuals:
| Polychaeta: |
Hartmann-Schröder (1996). Die Tierwelt Deutschlands: Annelida, Borsten würmer, Polychaeta, Teil 58. IMIS |
| Amphipoda: |
Roger J. Lincoln (1979). British Marine Amphipoda: Gammaridae. IMIS |
| Cumacea: |
N.S. Jones (1976). British Cumaceans. IMIS |
| Brachyura: |
J.P.H.M. Adema (1991). Krabben van Nederland en België. IMIS |
| Pycnogonida: |
P.E. King (1974). British Seaspiders. IMIS |
| Isopoda: |
E. Naylor (1972). British Marine Isopods. IMIS |
| Bivalvia: |
Norman Tebble (1976). British Bivalve Seashells. IMIS
R.H. de Bruyne (1994). Schelpen van de Nederlandse kust. IMIS |
| Decapoda: |
G. Smaldon (1979). British coastal shrimps and prawns (Caridea). IMIS
P.J. Hayward & J.S. Ryland (1996). General Handbook of the Marine Fauna of North-West Europe. IMIS |
Sampling methodology (period 1976-1986)
| Scientist |
Van Veen(m²) |
Volume sample (l) |
Immediately fixed |
Fixation (% formalin) |
Replicates |
Sieve mesh size µm |
Gutter |
Sediment # sieves |
| Vanosmael, C. (1977) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
870 |
|
19 |
| Van Steen, E. (1978) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
870 |
Yes |
17 |
| Rappé, G. (1978) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
870 |
|
17-18-19 |
| Kerckhof, F. (1980) |
0.1026 |
min. 5 |
Yes |
7 |
3-2 |
870 |
|
15 |
| Meheus, L. (1981) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
250 |
Yes |
9 |
| De Rycke, R. (1982) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
870 |
Yes |
9 |
| Vanosmael, C. (1982) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
250 |
|
|
| Waeterschoot, H. (1984) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
870 |
Yes |
8 |
| Brendonck, L. (1985) |
0.1026 |
min. 5 |
Yes |
7 |
3 |
870 |
Yes |
15 |
| Wittoeck, J. |
0.1026 |
min. 5 |
Yes |
7 |
1-3 |
1000 |
|
|
(0.12) |
min. 5 |
Yes |
7 |
1-10 |
1000 |
Yes |
7 |
Sampling methodology (period 1994-2001)
| Scientist |
Van Veen(m²) |
Volume sample (l) |
Immediately fixed |
Fixation (% formalin) |
Replicates |
Sieve mesh size µm |
Gutter |
Sediment # sieves |
| Coenjaerts, J. (1997) |
0.1026 |
min 5cm |
no |
7 |
1 |
1000 |
no |
Coulter |
| Philips, F. (1998) |
|
|
|
|
| Degraer, S. (1999) |
0.1026 |
5 |
no |
8 |
1 |
1000 |
no |
Coulter |
| Taverniers, K. (2000) |
0.12 |
min 5 |
no |
8 |
1 |
1000 |
no |
Coulter |
| Deneudt, K. (2000) |
0.1026 |
|
|
|
| Erdey, M. (2000) |
0.1026 |
|
no |
8 |
1 |
1000 |
no |
Coulter |
| Van Hoey, G. (2000) |
0.1/0.12 |
5 |
yes |
8 |
5 |
1000 |
no |
Coulter |
General sampling info
Possible disadvantages of the Van Veen grab:
- Fast moving organisms (e.g. some Brachyura, Mysida) can easily escape from the closing grab. Hence, the
number of mobile species that is found in the samples never constitutes the number of species actually
present on the sampled site.
- The maximum penetration depth in the sediment of the Van Veen grab is about 15 cm. This limited depth range
biases the sampling of bigger and deeper burrowing macrobenthos species such as Ensis sp., Lanice
conchilega, Venerupis senegalensis, etc).
- On harder substrates (e.g. very fine sands, places with large gravel fraction) the penetration is sometimes
limited to the upper centimetres of the sediment. As the catch efficiency is related to the penetration
depth of the grab, it is from the utmost importance that with every sample that is taken the penetration
depth should be kept to at least 5 cm. The latter corresponds with a minimum sediment volume of 5 l as was
assumed in former studies (see sampling methodology).
- The ideal impact position of the grab just before a sample is taken should be perpendicular to the bottom
surface. Strong currents or drifting of the vessel can jeopardise a good sampling. It is therefore advised
that in such cases the ship would cast anchor. Nevertheless it is imperative to check the contents of the
grab with every sampling and to repeat the sampling when falling short of the mentioned criteria.
- On every sampling occasion a sediment sample is taken (using a plastic tube or core)
- The entire contents of the Van Veen grab ends up on a PVC tray with a funnel like opening and is washed into
a bucket using seawater. That bucket is then emptied over a 1000 µm sieve and rinsed with seawater until
only the coarse sediment and the macrobenthos organisms remain. This residue is transferred into a recipient
and fixated with a neutralised (with seawater) formalin solution to a concentration of 7-8 %. As noticed in
tables 1 and 2 the described technique differs from the one used during the period 1977-1986. In the latter
the samples were firstly fixated in the buckets before pored over the 1000 µm (or 870 µm) sieve. A gutter
was often used for a better separation of biota and sediment.
Processing samples
Every sample (already stained with a Bengal Rose or eosin solution) is decanted (10 times) on a 1000 µm sieve in
order to separate the present fauna from the sediment. With this technique the residue of coarse sediment and
fauna is pored into a measuring cup (5 l) and is brought into suspension with a strong jet of water. During this
process the smaller and lighter organisms will go into suspension, whereas the heavier sediment and organisms
remain on the bottom. The water containing the organisms is there after pored over a 1000 µm sieve. All retained
fauna is stored in a 4-8 % neutral formalin solution and stained with Bengal-Rose of eosin (if not done
earlier). Both solutions colour all organic material. This enables easier sorting of the animals. After
decantation the residue is checked for bigger and heavier organisms (e.g. Mollusca, Actinaria).
Decantation must not be applied to fine sediment samples (< 1000 µm) as all sediment is directly rinsed through
the 1 mm sieve and what remains on the sieve can directly be fixated.
Identification
All organisms are counted and identified, if possible to species level. For some animal groups however this is
not the case:
- Nematoda, Nemertinea, Sipunculida, Turbellaria, Actinaria, Oligochaeta and Copepoda are never identified to
a higher level because they are not representative for the macrobenthos community. The sampling technique is
not suited to quantify these groups neither.
- The identification of some taxa is still under discussion. Whether they should be identified up to species
level or grouped into complexes is still not clear due to uncertain taxonomy (e.g. Phyllodoce maculata of P.
mucosa; Harmothoë spp.; Bathyporeia spp.; Cirratulidae; Capitellidae).
Sediment analysis
Two standard methods were applied:
- using sieves with different mesh sizes
During the period 1976-1986 all sediment analyses were done using at least 7 sieves with
different mesh size (see table 1). Approximately 25 g of homogenized substrate was used for grain size
analysis of the sand fraction according to Buchanan & Kain (1971). The wet-sieved fraction smaller than
63 µm was used as a measure for mud content.
- using a Coulter LS 100
From 1994 on all sediment samples were analysed using a Coulter counter. Within the measuring
range of 2 µm to 850 µm the grain sizes are measured. The fraction larger than 850 µm is separated and
expressed as weighed percentage. The subdivision in sediment fractions follows the Wentworth scale
(Buchanan, 1984).
Density
All densities are shown as the number of individuals per square meter (ind/m²). Only the anterior parts of
the organisms are counted.
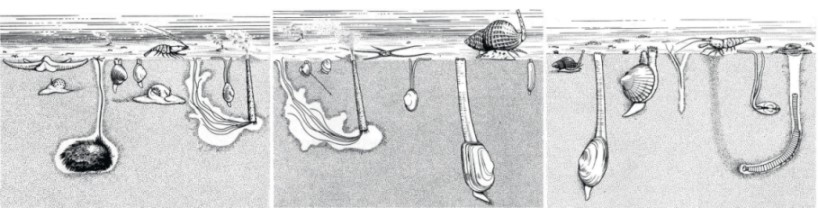
Definition macrobenthos
Macrobenthos is considered to be all organisms living in the bottom of the sea and retained on a sieve with a
mesh size of 1 mm. These organisms are mainly bristle worms (Polychaeta), bivalves (Mollusca), echinoderms
(Echinodermata) and amphipods (Crustacea).
|


