Cordylophora caspia - Ponto-Caspian freshwater hydroid
SCIENTIFIC NAME
Cordylophora caspia (Pallas, 1771)
The freshwater hydroid C. caspia originates from the Black Sea and Caspian Sea region [2].
This hydroid can live in fresh and brackish water, including estuaries, lagoons, rivers, canals and lakes. The species prefers shady places and occurs predominantly at depths of 0-10 metres. It attaches itself to a hard substrate, such as rocks, wooden shelves, boats, shells and submerged aquatic plants [2-4].
First observation in Belgium
The first observation of the freshwater hydroid in Belgium was in Nieuwpoort (1905). Here, several colonies with a height of five centimetres were found. These were attached to planks that floated in the port of Nieuwpoort for two and a half months [5].
Spreading in Belgium
In 1946, the presence of the freshwater hydroid near Ostend was reported [6]. A book written in 1952 states that this species is common in the brackish waters along the coast of the North Sea, including the coast of Belgium [3]. In 2002 and 2006, the species was seen respectively in the Dievegat creek near Zwin Nature Park and close to Nieuwpoort [7].
Spreading in neighbouring countries
The freshwater hydroid spread to western Europe from the Black Sea and the Caspian Sea through canals, lakes and rivers. From there, three different routes were taken: a northern, central and southern route [2] (figure 1). Via the northern route, the freshwater hydroid ended up in the Baltic Sea. Here, this cnidarian was first noticed in 1816, near the Swedish coast [8]. The migration from the Black and Caspian Sea to the far west of Europe occurred mainly via the central route [9]. This route runs through the rivers and canals of Ukraine and Poland to northern Germany [2]. This freshwater hydroid was observed in the German Elbe and the waters of the state of Schleswig in 1858 [8]. Via the southern route, this hydroid spread through rivers and canals from Romania, Hungary, Austria and Germany to the Netherlands [2]. The freshwater hydroid was first observed in Amsterdam in 1874 in the Amstel [6]. Nowadays, the species is widespread in the Netherlands [4, 10].
In France, the freshwater hydroid was not observed until 1901. That year, it was present in the Loire estuary in the northeast of the Bay of Biscay [11]. By 1946, the species was present worldwide, including the United Kingdom, Egypt, the United States, Brazil, New Zealand and China [6].
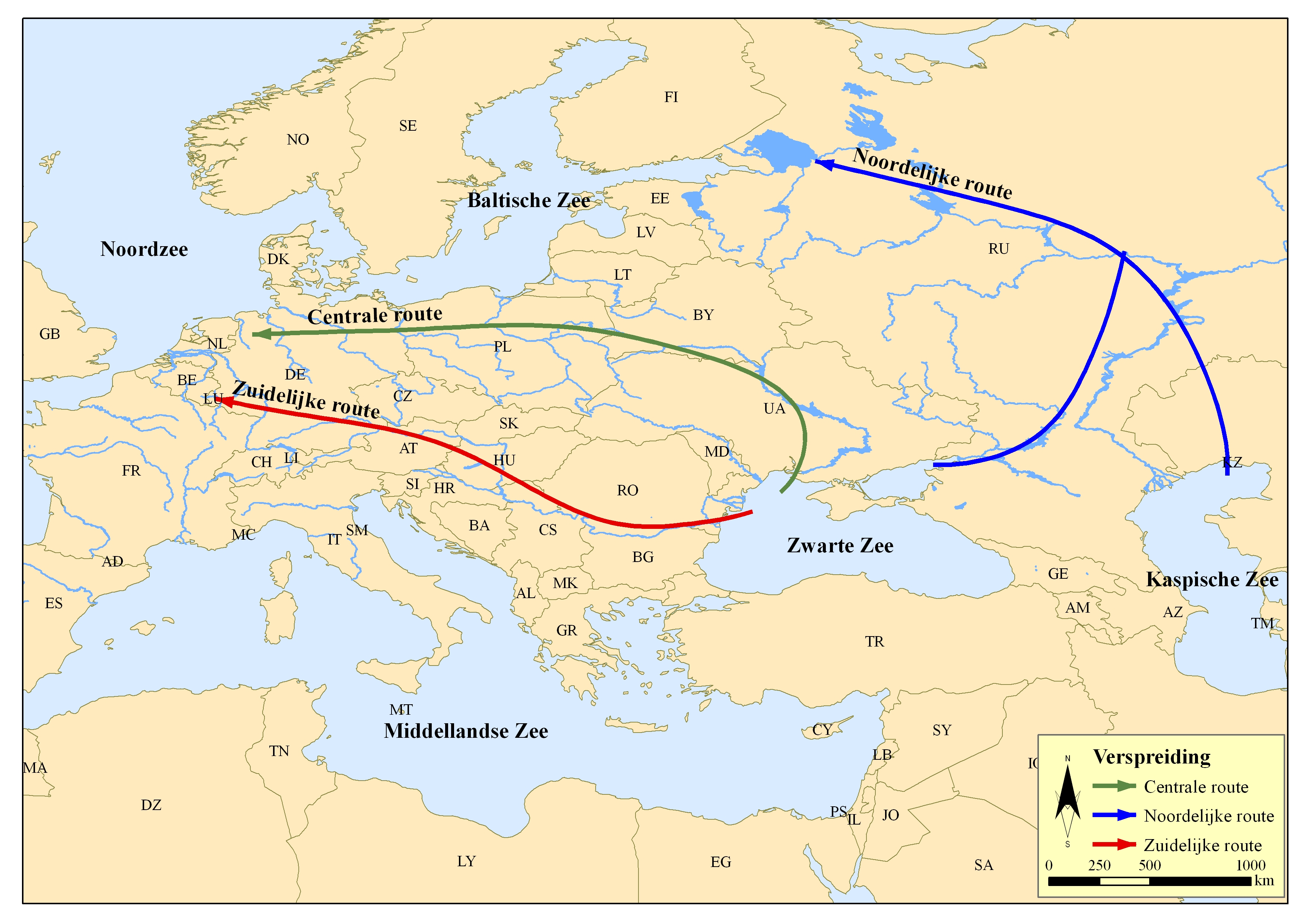
Figure 1: Dispersal of the freshwater hydroid from its area of origin to Europe. © VLIZ, after Bij de Vaate et al., 2002 [2].
The introduction of Ponto-Caspian species to European waters got facilitated by the construction of canals. Via these canals, the Caspian mud shrimp ended up in our region. The freshwater hydroid can spread through rivers by attaching to ship hulls, pieces of wood or floating plant material [2]. It is also possible that the species got imported via the ballast water of ships that sequentially goes to many different estuaries [3, 12].
The freshwater hydroid grows and reproduces best in brackish water, but the species can live and reproduce in fresh water and water with a salinity up to 35 PSU (seawater). A temperature between 10 and 28°C is ideal for this hydroid, but it can survive temperatures up to 35°C. Temperatures as low as -5°C can be overcome by this hydroid thanks to a resting stage, also called ‘menont’ by scientists. During the resting phase, most of the colony dies, while the soft tissues are withdrawn into a protective shell or perisarc. The hydroid cannot grow or reproduce during this survival stage. Once the unfavourable conditions are over, the hydroid returns to being a fully functional organism
Growth and reproduction only occur in eutrophic conditions. The rich fertilization of west European agricultural land and the associated discharge of nitrogen and phosphorus into canals and rivers explains the success of this species in our regions [8]. The freshwater hydroid propagates both sexually and asexually. For sexual reproduction, multiple reproductive organs or gonophores are formed, with 6 to 10 eggs each. Sperm gets released into the ocean and eggs get fertilized in the female gonophores, where the embryos develop into planulae (larvae) [13]. Asexual reproduction occurs through the formation of buds, in which small parts of the hydroid transform into new individuals while the parental hydroid breaks down. New hydroids can also form from the vertical branches of the attachment organ, also called the hydrorhiza [2]
The species does not have any specific requirements for the type of substrate to which it attaches. The only condition is that the surface is hard [6].
By attaching to a floating surface (e.g. boat, floating plank etc.), the freshwater hydroid can easily reach new areas [3]. If the hydroid is in its resting state, it is resistant to drought and extreme or highly fluctuating temperatures [2]. During the resting stage, the hydroid can stick to the legs and feathers of aquatic birds and get transported to new areas [3].
Although the freshwater hydroid lives attached to a substrate, its larvae move freely in the water column and can thus spread through rivers and canals. The larvae develop on the reproductive organs of the female hydroid and can roam freely in the water column for four to five weeks before they attach themselves [2, 8]. When these larvae end up in ships' ballast water, they can get transported over large distances. The latter explains the worldwide but discontinuous distribution of the freshwater hydroid [2].
Colonies develop well in water with salinity ranging from almost fresh (0.3 PSU) to brackish (10 PSU) conditions but can tolerate a range in salinity of 0.08 PSU to 35 PSU (seawater), although the colonies appear less healthy in these conditions [6]
It is well-known that dense colonies of freshwater hydroids change the structure of the soil. Therefore, an impact is to be expected on the biotic communities of both the soil and in the water column [14, 15]. For example, some animals find protection from predators or strong currents between the packed colonies of this hydroid. These species can become more successful in the presence of the freshwater hydroid. The dense hydroid colonies also absorb a lot of suspension material, hence, depriving native species of food. Furthermore, the hydroid colonies themselves form a food source for many other species. They can compete with native species for space on hard substrates.
In general, the presence of the freshwater hydroid and other non-native species, mainly from the Ponto-Caspian region, prevent the recovery of native species. For example, during the last thirty years, the water quality of the Rhine in Germany has been steadily recovering, but the original species composition has not yet returned due to the presence of non-native species [16].
The freshwater hydroid is the most common biofouling species in the port of Antwerp, where it causes some problems. This organism clogs the water pipes of port companies that pump up cooling water from the Scheldt. Cooling water installations are an attractive environment for the freshwater hydroid: there is a constant supply of oxygen and food in the pipes [17] and the predation pressure is limited [18]. Currently, many Antwerp port companies use chlorine to fight this invasive species. However, this is not obvious since the freshwater hydroid is a tough species and can easily regenerate again after exposure to chlorine treatment. To control its growth and reproduction, the use of biocides should regularly be re-executed without exceeding the current discharge standards. Another method of control is sporadic exposure to hot water. This changes the environmental conditions that are otherwise very attractive for the hydroid. This alternative way of control allows the elimination of the hydroid without posing a risk to the environment [19]. Precisely because of its strong resistance, it may not be possible to completely eradicate this species in the port areas [20].
The freshwater hydroid forms colonies and has a brown to yellowish colour. Colonies grow 10 cm high. The freshwater hydroid varies in shape, size, number of branches and number of reproductive organs depending on the environment. Mainly the salinity, but also the temperature and light, play an important role in this [2, 3, 6].
The main stem of a colony is also called the hydrocaulus. The hydrocaulus is connected to the basal attachment system (figure 2), called the hydrorhiza, by which the colony attaches itself to a solid substrate. The side branches of the hydrocaulus, or hydrocladia, are in turn branched with pedicels. The hydrocladia have a thick exterior layer, called the perisarc (figure 2). At the end of each pedicel, there is a single polyp, called the hydranth. All hydrants within one colony are either female or male. Each hydranth has a mouth surrounded by tentacles [21, 22].
The freshwater hydroid can be distinguished from other hydroids based on several characteristics. For instance, this species forms large colonies, with the hydranths located at the ends of the hydrocladia, the pedicel. The tentacles are spread across the entire hydranth and are not grouped in one place, as in many other species (including the hydroid Garveia franciscana) [21].
The tentacles contain nematocysts used to defend themselves and collect food. Only a light touch of the tentacles is needed to fire the coiled-up wire from the nematocysts at potential prey. The wire contains paralyzing poison [22].
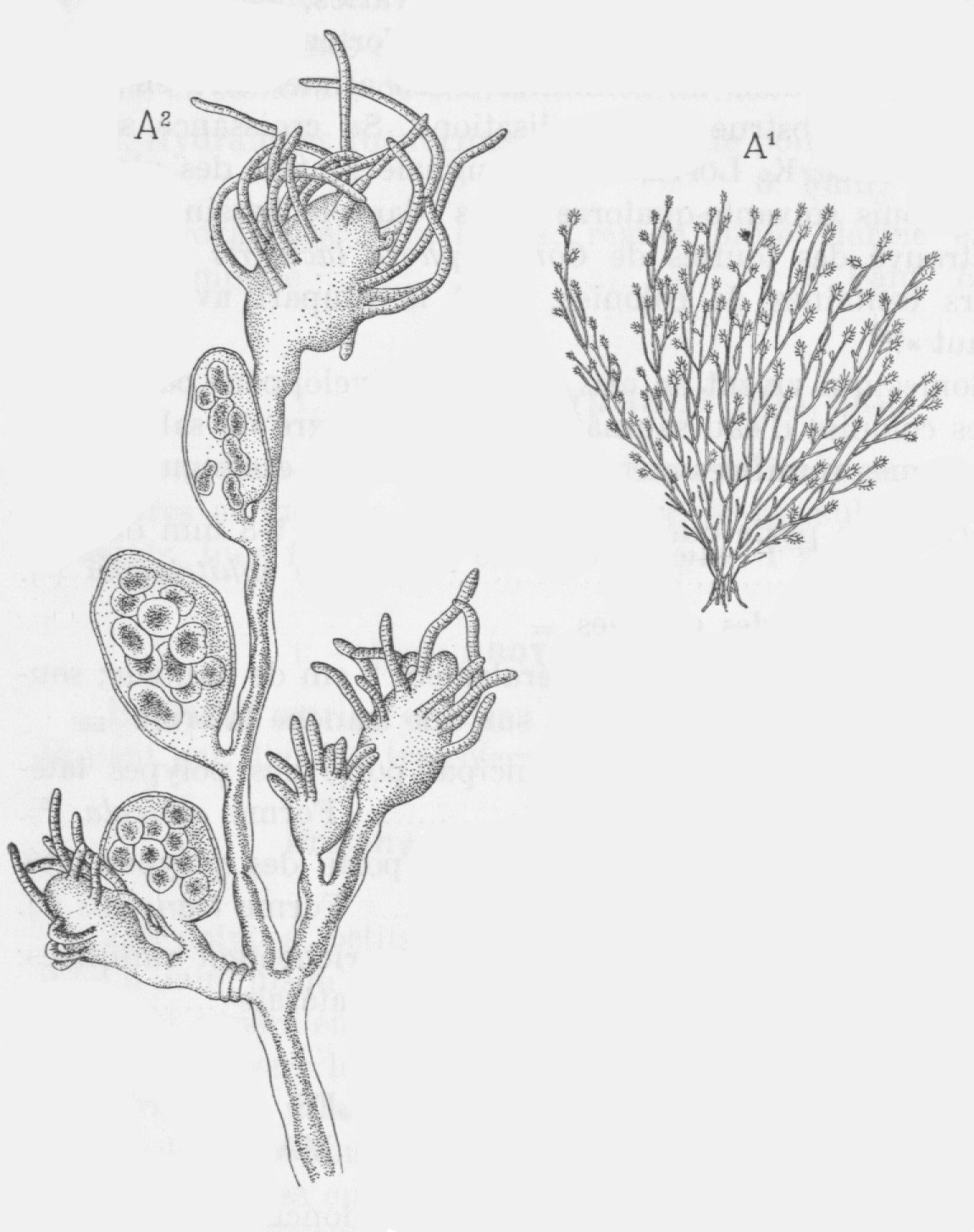
Figure 2: Detailed drawing of a hydroid (Source: Leloup, 1952) [3].
[1] World Register of Marine Species (WoRMS) (2020). Cordylophora caspia (Pallas, 1771). [http://www.marinespecies.org/aphia.php?p=taxdetails&id=117428] (2020-11-17).
[2] Bij de Vaate, A.; Jazdzewski, K.; Ketelaars, H.A.M.; Gollasch, S.; van der Velde, G. (2002). Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can. J. Fish. Aquat. Sci./J. Can. Sci. Halieut. Aquat. 59(7): 1159-1174. [http://www.vliz.be/en/imis?module=ref&refid=127047]
[3] Leloup, E. (1952). Coelentérés. Faune de Belgique. Institut royal des Sciences naturelles de Belgique: Brussels, Belgium. 283 pp. [http://www.vliz.be/en/imis?module=ref&refid=125914]
[4] Vervoort, W. (1964). Notes on the distribution of Garveia franciscana (Torrey, 1902) and Cordylophora caspia (Pallas, 1771) in the Netherlands. Zool. Meded. 39: 125-146. [http://www.vliz.be/en/imis?module=ref&refid=110855]
[5] Loppens, K. (1905). Animaux marins vivant dans l'eau saumâtre. Ann. Soc. R. Zool. Malacol. Bel. 40: VII-VIII. [http://www.vliz.be/en/imis?module=ref&refid=70032]
[6] Vervoort, W. (1946). Hydrozoa (C1): A. Hydropolypen. Fauna van Nederland, 14. A.W. Sijthoff's Uitgeversmaatschappij NV: Leiden, The Netherlands. 336 pp. [www.vliz.be/en/imis?module=ref&refid=39507]
[7] Waarnemingen afkomstig van Waarnemingen.be: een initiatief van Natuurpunt Studie vzw en de Stichting Natuurinformatie (2018). Brakwaterpoliep - Cordylophora caspia (Pallas, 1771). [https://waarnemingen.be/soort/view/27067?waardplant=0&poly=1&from=2000-08-08&to=2018-08-08&method=0&rar=0&only_approved=0&maand=0&prov=0&rows=20&os=0&hide_hidden=0&hide_hidden=1&show_zero=0] (2018-08-08).
[8] Nehring, S.; Leuchs, H. (1999). Neozoa (Makrozoobenthos) an der deutschen Nordseeküste: eine Übersicht. Bericht BfG, 1200. Bundesanstalt für Gewässerkunde = Federal Institute of Hydrology: Koblenz. 131 pp. [http://www.vliz.be/en/imis?module=ref&refid=120661]
[9] Kinzelbach, R. (1995). Neozoans in European waters - exemplifying the worldwide process of invasion and species mixing. Experientia 51(5): 526-538. [http://www.vliz.be/en/imis?module=ref&refid=206332]
[10] Van der Velde, G.; Nagelkerke, I.; Rajagopal, S.; Bij de Vaate, A. (2002). Invasions by alien species in inland freshwater bodies in western Europe: the Rhine Delta, in: Leppäkoski, E. et al. Invasive aquatic species of Europe: distribution, impacts and management. Kluwer Academic: Dordrecht: pp. 360-672. [http://www.vliz.be/en/imis?module=ref&refid=40616]
[11] Goulletquer, P.; Bachelet, G.; Sauriau, P.G.; Noel, P. (2002). Open Atlantic coast of Europe: a century of introduced species, in: Leppäkoski, E. et al. Invasive aquatic species of Europe: Distribution, impacts and management. Kluwer Academic: Dordrecht: pp. 276-290. [http://www.vliz.be/en/imis?module=ref&refid=40609]
[12] Funke, H.C. (1922). Hydroiden, in: Redeke, H.C. Flora en fauna der Zuiderzee: Monografie van een brakwatergebied. C. De Boer Jr.: Den Helder: pp. 185-210. [http://www.vliz.be/en/imis?module=ref&refid=115198]
[13] Gili, J.-M.; Hughes, R. (1995). The ecology of marine benthic hydroids. Oceanogr. Mar. Biol. Ann. Rev. 33: 351-426. [http://www.vliz.be/nl/catalogus?module=ref&refid=123898]
[14] Olenin, S.; Leppäkoski, E. (1999). Non-native animals in the Baltic Sea: alteration of benthic habitats in coastal inlets and lagoons. Hydrobiologia 393: 233-243. [http://www.vliz.be/en/imis?module=ref&refid=127049]
[15] Leppäkoski, E.; Gollasch, S.; Gruszka, P.; Ojaveer, H.; Olenin, S.; Panov, V. (2002). The Baltic: a sea of invaders. Can. J. Fish. Aquat. Sci./J. Can. Sci. Halieut. Aquat. 59(7): 1175-1188. [http://www.vliz.be/nl/catalogus?module=ref&refid=28743]
[16] Bernauer, D.; Jansen, W. (2006). Recent invasions of alien macroinvertebrates and loss of native species in the upper Rhine River, Germany. Aquat. Invasions 1(2): 55-71. [http://www.vliz.be/nl/catalogus?module=ref&refid=300125]
[17] Boero, F. (1984). The ecology of marine hydroids and effects of environmental factors: a review. Mar. Ecol. 5: 93-118. [http://www.vliz.be/en/imis?module=ref&refid=127052]
[18] Roos, P.J. (1979). Two-stage life cycle of a Cordylophora population in the Netherlands. Hydrobiologia 62(3): 231-239. [http://www.vliz.be/en/imis?module=ref&refid=127055]
[19] Folino-Rorem, N.C.; Indelicato, J. (2005). Controlling biofouling caused by the colonial hydroid Cordylophora caspia. Wat. Res. 39(12): 2731-2737. [http://www.vliz.be/nl/catalogus?module=ref&refid=300126]
[20] Verween, A. (2018). Persoonlijke mededeling
[21] Hayward, P.J.; Ryland, J.S. (1995). Handbook of the marine fauna of North-West Europe. Oxford University Press: Oxford, UK. ISBN 0-19-854054-X. XI. 800 pp. [http://www.vliz.be/en/imis?module=ref&refid=10501]
[22] Faasse, M. (2019). Persoonlijke mededeling
[23] Ruppert, E.E.; Barnes, R.D. (1994). Invertebrate zoology. 6th edition. Saunders College Publishing: Orlando. ISBN 0-03-026668-8. 1056 pp. [http://www.vliz.be/nl/catalogus?module=ref&refid=9414]
VLIZ Alien Species Consortium (2020). Cordylophora caspia – Freshwater hydroid. Non-indigenous species in the Belgian part of the North Sea and adjacent estuaries anno 2020. Flanders Marine Institute (VLIZ). 8 pp.

